
Topic 14 - Chemical bonding and structure (HL)
Question 1
HLPaper 1Which combination correctly describes the geometry of BrF4-?

Question 2
HLPaper 1How many sigma (σ) and pi (π) bonds are present in this molecule?


Question 3
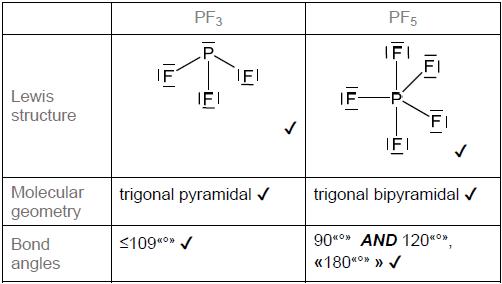
HLPaper 2Lewis (electron dot) structures are useful models.
Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory todeduce the molecular geometry of each species including bond angles.
Predict whether the molecules PF3 and PF5 are polar or non-polar.
State the type of hybridization shown by the phosphorus atom in PF3.
Question 4
HLPaper 2Compound A is in equilibrium with compound B.

Predict the electron domain and molecular geometries around the oxygen atom of molecule A using VSEPR

State the type of hybridization shown by the central carbon atom in molecule B.
State the number of sigma (σ) and pi (π) bonds around the central carbon atom in molecule B.

The IR spectrum of one of the compounds is shown:

COBLENTZ SOCIETY. Collection © 2018 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Deduce, giving a reason, the compound producing this spectrum.
Compound A and B are isomers. Draw two other structural isomers with the formula C3H6O.
The equilibrium constant, Kc, for the conversion of A to B is 1.0×108 in water at 298 K.
Deduce, giving a reason, which compound, A or B, is present in greater concentration when equilibrium is reached.
f(i).
Calculate the standard Gibbs free energy change, ∆G⦵, in kJ mol–1, for the reaction (A to B) at 298 K. Use sections 1 and 2 of the data booklet.
f(ii).
Propanone can be synthesized in two steps from propene.Suggest the synthetic route including all the necessary reactants and steps.
g(i).
Propanone can be synthesized in two steps from propene.
Suggest why propanal is a minor product obtained from the synthetic route in (g)(i).
g(ii).
Question 5
HLPaper 2There is concern about damage done to the ozone layer in the stratosphere by jet-propelledaircraft.
Formulate two equations to show how nitrogen(II) oxide, NO, catalyses the destructionof ozone.
Suggest why the loss of ozone is an international environmental concern.
Question 6
HLPaper 1What is the formal charge of the oxygen atom in H3O+?
Question 7
HLPaper 2Butanoic acid, CH3CH2CH2COOH, is a weak acid and ethylamine, CH3CH2NH2, is a weak base.
State the equation for the reaction of each substance with water.

Draw a diagram showing the delocalization of electrons in the conjugate base of butanoic acid.
Deduce the average oxidation state of carbon in butanoic acid.
A 0.250 mol dm−3 aqueous solution of butanoic acid has a concentration of hydrogen ions, [H+], of 0.00192 mol dm−3. Calculate the concentration of hydroxide ions, [OH−], in the solution at 298 K.
Determine the pH of a 0.250 mol dm−3 aqueous solution of ethylamine at 298 K, using section 21 of the data booklet.
Sketch the pH curve for the titration of 25.0 cm3 of ethylamine aqueous solution with 50.0 cm3 of butanoic acid aqueous solution of equal concentration. No calculations are required.

Explain why butanoic acid is a liquid at room temperature while ethylamine is a gas at room temperature.
State a suitable reagent for the reduction of butanoic acid.
Deduce the product of the complete reduction reaction in (e)(i).
Question 8
HLPaper 1Which statement is correct?
Question 9
HLPaper 1Which combination describes the PH4+ ion?

Question 10
HLPaper 2Copper forms two chlorides, copper(I) chloride and copper(II) chloride.
Two electrolysis cells were assembled using graphite electrodes and connected in series as shown.

Copper(I) chloride undergoes a disproportionation reaction, producing copper(II) chloride and copper.
2Cu+ (aq) → Cu (s) + Cu2+ (aq)
Dilute copper(II) chloride solution is light blue, while copper(I) chloride solution is colourless.
State the electron configuration of the Cu+ ion.
a(i).
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, Δ_H_θ, in kJ, for this reaction, using section 12 of the data booklet.
a(ii).
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label _E_a (cat).

a(iii).
Explain how the catalyst increases the rate of the reaction.
a(iv).
Solid copper(II) chloride absorbs moisture from the atmosphere to form a hydrate of formula CuCl2•xH2O.
A student heated a sample of hydrated copper(II) chloride, in order to determine the value of x. The following results were obtained:
Mass of crucible = 16.221 g
Initial mass of crucible and hydrated copper(II) chloride = 18.360 g
Final mass of crucible and anhydrous copper(II) chloride = 17.917 g
Determine the value of x.
State how current is conducted through the wires and through the electrolyte.
Wires:
Electrolyte:
c(i).
Write the half-equation for the formation of gas bubbles at electrode 1.
c(ii).
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
c(iii).
Deduce the half-equation for the formation of the gas identified in (c)(iii).
c(iv).
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24 of the data booklet.
e(i).
Comment on the spontaneity of the disproportionation reaction at 298 K.
e(ii).
Calculate the standard Gibbs free energy change, Δ_G_θ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
e(iii).
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
e(iv).
Deduce, giving a reason, the sign of the standard enthalpy change, Δ_H_θ, for the disproportionation reaction at 298 K.
e(v).
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
e(vi).
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
f(i).
Deduce why the Cu(I) solution is colourless.
f(ii).
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
f(iii).
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
f(iv).
