
Topic 6 - Chemical kinetics
Question 1
SLPaper 1Several reactions of calcium carbonate with dilute hydrochloric acid are carried out at the same temperature.
CaCO3 (s) + 2HCl (aq) → CaCl2 (aq) + H2O (l) + CO2 (g)
Which reaction has the greatest rate?

Question 2
SLPaper 1Samples of sodium carbonate powder were reacted with separate samples of excess hydrochloric acid.
Na2CO3 (s) + 2HCl (aq) → CO2 (g) + 2NaCl (aq) + H2O (l)
Reaction I: 1.0 g Na2CO3 (s) added to 0.50 mol dm−3 HCl (aq)
Reaction II: 1.0 g Na2CO3 (s) added to 2.0 mol dm−3 HCl (aq)
What is the same for reactions I and II?
Question 3
SLPaper 1Why does a reaction for a sample of gases, at constant temperature, occur faster at higher pressure?
Question 4
SLPaper 2Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
Methanol is usually manufactured from methane in a two-stage process.
CH4 (g) + H2O (g) ⇌ CO (g) + 3H2 (g)
CO (g) + 2H2 (g) ⇌ CH3OH (l)
Consider the first stage of the reaction.
CH4 (g) + H2O (g) ⇌ CO (g) + 3H2 (g)
The diagram shows the Maxwell-Boltzmann curve for the uncatalyzed reaction.
Draw a distribution curve at a lower temperature (T2) and show on the diagram how the addition of a catalyst enables the reaction to take place more rapidly than at T1.

The hydrogen peroxide could cause further oxidation of the methanol. Suggest a possible oxidation product.
Determine the overall equation for the production of methanol.
c(i).
8.00 g of methane is completely converted to methanol. Calculate, to three significant figures, the final volume of hydrogen at STP, in dm3. Use sections 2 and 6 of the data booklet.
c(ii).
Determine the enthalpy change, Δ_H_, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol−1.
d(i).
State the expression for _K_c for this stage of the reaction.
d(ii).
State and explain the effect of increasing temperature on the value of Kc.
d(iii).
Question 5
SLPaper 1On the following Maxwell-Boltzmann distribution, which letter represents activation energy?

Question 6
HLPaper 2Copper forms two chlorides, copper(I) chloride and copper(II) chloride.
Two electrolysis cells were assembled using graphite electrodes and connected in series as shown.

Copper(I) chloride undergoes a disproportionation reaction, producing copper(II) chloride and copper.
2Cu+ (aq) → Cu (s) + Cu2+ (aq)
Dilute copper(II) chloride solution is light blue, while copper(I) chloride solution is colourless.
State the electron configuration of the Cu+ ion.
a(i).
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, Δ_H_θ, in kJ, for this reaction, using section 12 of the data booklet.
a(ii).
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label _E_a (cat).

a(iii).
Explain how the catalyst increases the rate of the reaction.
a(iv).
Solid copper(II) chloride absorbs moisture from the atmosphere to form a hydrate of formula CuCl2•xH2O.
A student heated a sample of hydrated copper(II) chloride, in order to determine the value of x. The following results were obtained:
Mass of crucible = 16.221 g
Initial mass of crucible and hydrated copper(II) chloride = 18.360 g
Final mass of crucible and anhydrous copper(II) chloride = 17.917 g
Determine the value of x.
State how current is conducted through the wires and through the electrolyte.
Wires:
Electrolyte:
c(i).
Write the half-equation for the formation of gas bubbles at electrode 1.
c(ii).
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
c(iii).
Deduce the half-equation for the formation of the gas identified in (c)(iii).
c(iv).
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24 of the data booklet.
e(i).
Comment on the spontaneity of the disproportionation reaction at 298 K.
e(ii).
Calculate the standard Gibbs free energy change, Δ_G_θ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
e(iii).
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
e(iv).
Deduce, giving a reason, the sign of the standard enthalpy change, Δ_H_θ, for the disproportionation reaction at 298 K.
e(v).
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
e(vi).
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
f(i).
Deduce why the Cu(I) solution is colourless.
f(ii).
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
f(iii).
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
f(iv).
Question 7
HLPaper 3Bromine and methanoic acid react in aqueous solution.
Br2 (aq) + HCOOH (aq) → 2Br− (aq) + 2H+ (aq) + CO2 (g)
The reaction was monitored by measuring the volume of carbon dioxide produced as time
progressed.
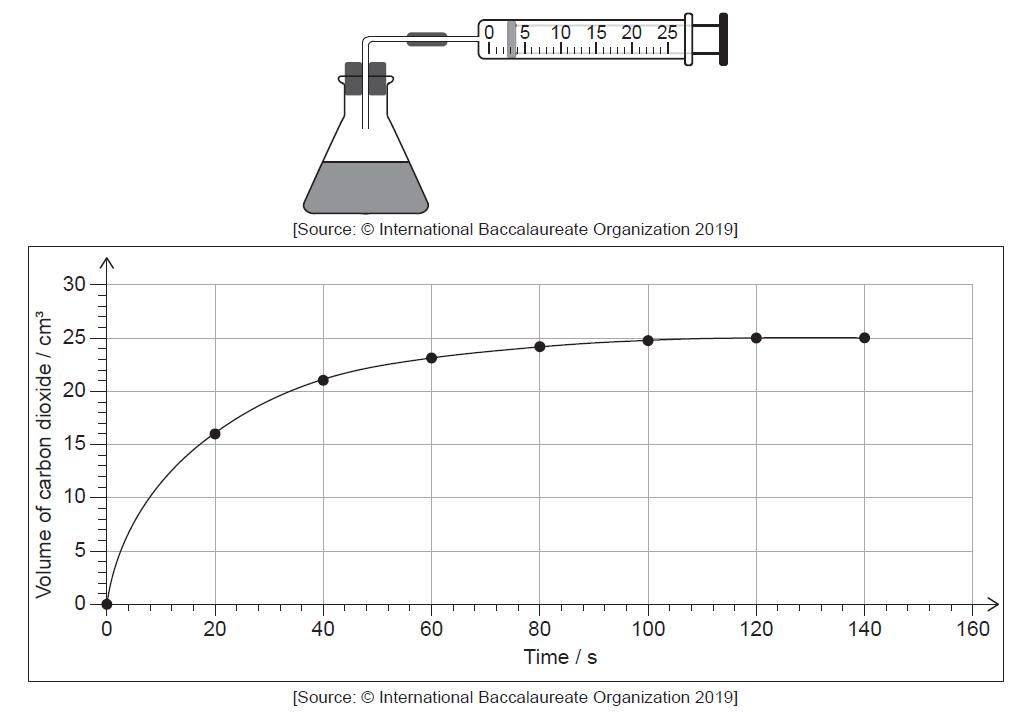
Determine from the graph the rate of reaction at 20 s, in cm3 s−1, showing your working.
Outline, with a reason, another property that could be monitored to measure the rate of this reaction.
Describe one systematic error associated with the use of the gas syringe, and how the error affects the calculated rate.
c(i).
Identify one error associated with the use of an accurate stopwatch.
c(ii).
Question 8
SLPaper 2Calcium carbonate reacts with hydrochloric acid.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
The results of a series of experiments in which the concentration of HCl was varied areshown below.
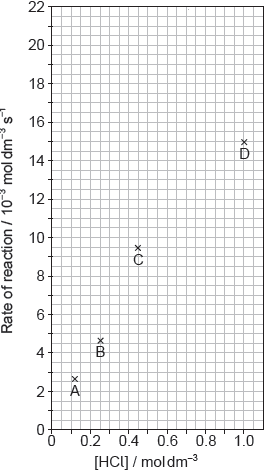
Outline two ways in which the progress of the reaction can be monitored. No practicaldetails are required.
Suggest why point D is so far out of line assuming human error is not the cause.
Suggest the relationship that points A, B and C show between the concentrationof the acid and the rate of reaction.
Question 9
SLPaper 1The potential energy profile for the reversible reaction, X + Y ⇌ Z is shown.
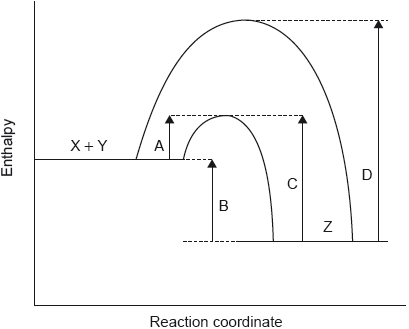
Which arrow represents the activation energy for the reverse reaction, Z → X + Y, with a catalyst?
Question 10
SLPaper 1Which arrow shows the activation energy of the uncatalysed forward reaction for this equilibrium?
2SO2 g+O2 g⇌2SO3 g ∆H=-196 kJ mol-1

