
Topic 19 - Electrochemical cells (HL)
Question 1
HLPaper 2Oxidation and reduction reactions can have a variety of commercial uses.
A student decides to build a voltaic cell consisting of an aluminium electrode, Al (s), a tin electrode, Sn (s), and solutions of aluminium nitrate, Al(NO3)3 (aq) and tin(II) nitrate, Sn(NO3)2 (aq).
Electron flow is represented on the diagram.
Label each line in the diagram using section 25 of the data booklet.

Write the equation for the expected overall chemical reaction in (a).
Calculate the cell potential using section 24 of the data booklet.
Calculate the Gibbs free energy change, Δ_G_⦵, in kJ, for the cell, using section 1 of the data booklet.
Question 2
HLPaper 2Magnetite, Fe3O4, is another ore of iron that contains both Fe2+ and Fe3+.
Iron exists as several isotopes.
Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
State the type of spectroscopy that could be used to determine their relative abundances.
b(i).
State the number of protons, neutrons and electrons in each species.

b(ii).
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
A voltaic cell is set up between the Fe2+ (aq) | Fe (s) and Fe3+ (aq) | Fe2+ (aq) half-cells.
Deduce the equation and the cell potential of the spontaneous reaction. Use section 24 of the data booklet.

The figure shows an apparatus that could be used to electroplate iron with zinc. Label the figure with the required substances.

Outline why, unlike typical transition metals, zinc compounds are not coloured.
Transition metals like iron can form complex ions. Discuss the bonding between transition metals and their ligands in terms of acid-base theory.
Question 3
HLPaper 1Consider the following table of standard electrode potentials.

Which is the strongest oxidizing agent?
Question 4
HLPaper 1What are the major products of electrolysing concentrated aqueous potassium iodide, KI(aq)?
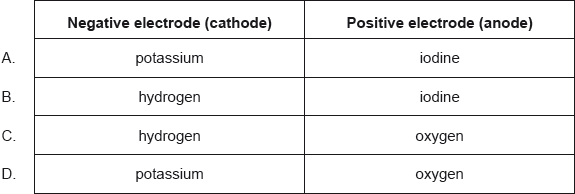
Question 5
HLPaper 2Copper forms two chlorides, copper(I) chloride and copper(II) chloride.
Two electrolysis cells were assembled using graphite electrodes and connected in series as shown.

Copper(I) chloride undergoes a disproportionation reaction, producing copper(II) chloride and copper.
2Cu+ (aq) → Cu (s) + Cu2+ (aq)
Dilute copper(II) chloride solution is light blue, while copper(I) chloride solution is colourless.
State the electron configuration of the Cu+ ion.
a(i).
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, Δ_H_θ, in kJ, for this reaction, using section 12 of the data booklet.
a(ii).
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label _E_a (cat).

a(iii).
Explain how the catalyst increases the rate of the reaction.
a(iv).
Solid copper(II) chloride absorbs moisture from the atmosphere to form a hydrate of formula CuCl2•xH2O.
A student heated a sample of hydrated copper(II) chloride, in order to determine the value of x. The following results were obtained:
Mass of crucible = 16.221 g
Initial mass of crucible and hydrated copper(II) chloride = 18.360 g
Final mass of crucible and anhydrous copper(II) chloride = 17.917 g
Determine the value of x.
State how current is conducted through the wires and through the electrolyte.
Wires:
Electrolyte:
c(i).
Write the half-equation for the formation of gas bubbles at electrode 1.
c(ii).
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
c(iii).
Deduce the half-equation for the formation of the gas identified in (c)(iii).
c(iv).
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24 of the data booklet.
e(i).
Comment on the spontaneity of the disproportionation reaction at 298 K.
e(ii).
Calculate the standard Gibbs free energy change, Δ_G_θ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
e(iii).
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
e(iv).
Deduce, giving a reason, the sign of the standard enthalpy change, Δ_H_θ, for the disproportionation reaction at 298 K.
e(v).
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
e(vi).
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
f(i).
Deduce why the Cu(I) solution is colourless.
f(ii).
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
f(iii).
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
f(iv).
Question 6
HLPaper 2The standard electrode potential of zinc can be measured using a standard hydrogen electrode (SHE).
Draw and annotate the diagram to show the complete apparatus required to measure the standard electrode potential of zinc.

Question 7
HLPaper 23.26 g of iron powder are added to 80.0 cm3 of 0.200 mol dm−3 copper(II) sulfate solution. The following reaction occurs:
Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s)
Determine the limiting reactant showing your working.
The mass of copper obtained experimentally was 0.872 g. Calculate the percentage yield of copper.
The reaction was carried out in a calorimeter. The maximum temperature rise of the solution was 7.5 °C.
Calculate the enthalpy change, Δ_H_, of the reaction, in kJ, assuming that allthe heat released was absorbed by the solution. Use sections 1 and 2 of thedata booklet.
State another assumption you made in (b)(i).
The only significant uncertainty is in the temperature measurement.
Determine the absolute uncertainty in the calculated value of Δ_H_ if the uncertainty in the temperature rise was ±0.2 °C.
Sketch a graph of the concentration of iron(II) sulfate, FeSO4, against time as the reaction proceeds.

Outline how the initial rate of reaction can be determined from the graph inpart (c)(i).
Explain, using the collision theory, why replacing the iron powder with a piece of iron of the same mass slows down the rate of the reaction.
A student electrolyzed aqueous iron(II) sulfate, FeSO4 (aq), using platinum electrodes. State half-equations for the reactions at the electrodes, using section 24 of thedata booklet.

Question 8
HLPaper 1Two cells undergoing electrolysis are connected in series.
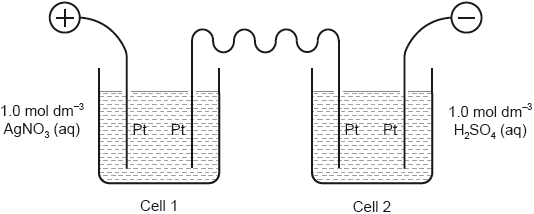
If xg of silver are deposited in cell 1, what volume of oxygen, in dm3 at STP, is given off in cell 2?
_A_r(Ag) = 108; Molar volume of an ideal gas at STP = 22.7 dm3 mol−1
Question 9
HLPaper 2An aqueous solution of silver nitrate, AgNO3 (aq), can be electrolysed using platinum electrodes.
Formulate the half-equations for the reaction at each electrode during electrolysis.
Cathode (negative electrode):
Anode (positive electrode):
Question 10
HLPaper 2The diagram shows an incomplete voltaic cell with a light bulb in the circuit.

Identify the missing component of the cell and its function.
Deduce the half-equations for the reaction at each electrode when current flows.

Annotate the diagram with the location and direction of electron movement when current flows.
Calculate the cell potential, in V, using section 24 of the data booklet.
Determine the loss in mass of one electrode if the mass of the other electrode increases by 0.10 g.
