
Topic 7 - Equilibrium
Question 1
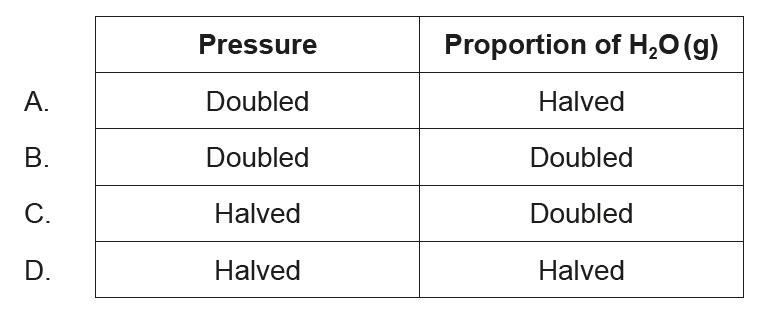
SLPaper 1Which changes produce the greatest increase in the percentage conversion of methane?
CH4 (g) + H2O (g) ⇌ CO (g) + 3H2 (g)
Question 2
SLPaper 2A molecule of citric acid, C6H8O7, is shown.

The equation for the first dissociation of citric acid in water is
C6H8O7 (aq) + H2O (l) ⇌ C6H7O7− (aq) + H3O+ (aq)
Identify a conjugate acid–base pair in the equation.
a(i).
The value of the equilibrium constant for the first dissociation at 298 K is 5.01 × 10−4.
State, giving a reason, the strength of citric acid.
a(ii).
The dissociation of citric acid is an endothermic process. State the effect on the hydrogen ion concentration, [H+], and on the equilibrium constant, of increasing the temperature.

a(iii).
Outline one laboratory methods of distinguishing between solutions of citric acid and hydrochloric acid of equal concentration, stating the expected observations.
Question 3
HLPaper 2Consider the following equilibrium reaction:
2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
State the equilibrium constant expression, _K_c, for the reaction above.
State and explain how the equilibrium would be affected by increasing the volume of the reaction container at a constant temperature.
SO2 (g), O2 (g) and SO3 (g) are mixed and allowed to reach equilibrium at 600 °C.

Determine the value of _K_c at 600 °C.
Question 4
HLPaper 2Compound A is in equilibrium with compound B.

Predict the electron domain and molecular geometries around the oxygen atom of molecule A using VSEPR

State the type of hybridization shown by the central carbon atom in molecule B.
State the number of sigma (σ) and pi (π) bonds around the central carbon atom in molecule B.

The IR spectrum of one of the compounds is shown:

COBLENTZ SOCIETY. Collection © 2018 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Deduce, giving a reason, the compound producing this spectrum.
Compound A and B are isomers. Draw two other structural isomers with the formula C3H6O.
The equilibrium constant, Kc, for the conversion of A to B is 1.0×108 in water at 298 K.
Deduce, giving a reason, which compound, A or B, is present in greater concentration when equilibrium is reached.
f(i).
Calculate the standard Gibbs free energy change, ∆G⦵, in kJ mol–1, for the reaction (A to B) at 298 K. Use sections 1 and 2 of the data booklet.
f(ii).
Propanone can be synthesized in two steps from propene.Suggest the synthetic route including all the necessary reactants and steps.
g(i).
Propanone can be synthesized in two steps from propene.
Suggest why propanal is a minor product obtained from the synthetic route in (g)(i).
g(ii).
Question 5
SLPaper 3The buffer formed by carbon dioxide, CO2(aq) and hydrogen carbonate ion, HCO3−(aq),plays an important role in maintaining the pH of blood.
Calculate the pH of the buffer from the following data and section 1 of the data booklet.
p_K_a(CO2) = 6.34
[HCO3−(aq)] = 1.40 × 10−2 mol dm−3
[CO2(aq)] = 1.25 × 10−3 mol dm−3
Explain the effect of a large amount of aspirin on the pH of blood.
Question 6
SLPaper 1Consider the reaction:
2N2O (g) ⇌ 2N2 (g) + O2 (g)
The values of _K_c at different temperatures are:

Which statement is correct at higher temperature?
Question 7
HLPaper 1What occurs when the pressure on the given equilibrium is increased at constant temperature?
N2(g) + O2(g) ⇌ 2NO(g) Δ_H_ = +180 kJ
Question 8
SLPaper 2Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
Methanol is usually manufactured from methane in a two-stage process.
CH4 (g) + H2O (g) ⇌ CO (g) + 3H2 (g)
CO (g) + 2H2 (g) ⇌ CH3OH (l)
Consider the first stage of the reaction.
CH4 (g) + H2O (g) ⇌ CO (g) + 3H2 (g)
The diagram shows the Maxwell-Boltzmann curve for the uncatalyzed reaction.
Draw a distribution curve at a lower temperature (T2) and show on the diagram how the addition of a catalyst enables the reaction to take place more rapidly than at T1.

The hydrogen peroxide could cause further oxidation of the methanol. Suggest a possible oxidation product.
Determine the overall equation for the production of methanol.
c(i).
8.00 g of methane is completely converted to methanol. Calculate, to three significant figures, the final volume of hydrogen at STP, in dm3. Use sections 2 and 6 of the data booklet.
c(ii).
Determine the enthalpy change, Δ_H_, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol−1.
d(i).
State the expression for _K_c for this stage of the reaction.
d(ii).
State and explain the effect of increasing temperature on the value of Kc.
d(iii).
Question 9
SLPaper 1Which factor does not affect the position of equilibrium in this reaction?
2NO2(g) ⇌ N2O4(g) Δ_H_ = −58 kJ mol−1
Question 10
HLPaper 2Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) ⇌ (H2N)2CO(g) + H2O(g) Δ_H_ < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
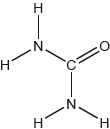
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
State the equilibrium constant expression, _K_c.
Predict, with a reason, the effect on the equilibrium constant, _K_c, when the temperature is increased.
Determine an approximate order of magnitude for _K_c, using sections 1 and 2 of the data booklet. Assume Δ_G_Θfor the forward reaction is approximately +50 kJ at 298 K.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
The mass spectrum of urea is shown below.
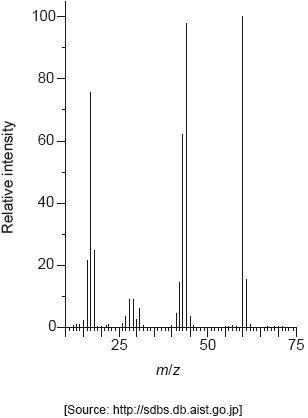
Identify the species responsible for the peaks at m/z = 60 and 44.

The IR spectrum of urea is shown below.
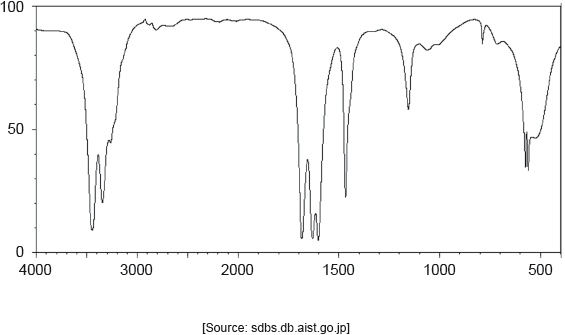
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.

Predict the number of signals in the 1H NMR spectrum of urea.
Predict the splitting pattern of the 1H NMR spectrum of urea.
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
