
Topic 17 - Equilibrium (HL)
Question 1
HLPaper 2Consider the following equilibrium reaction:
2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
State the equilibrium constant expression, _K_c, for the reaction above.
State and explain how the equilibrium would be affected by increasing the volume of the reaction container at a constant temperature.
SO2 (g), O2 (g) and SO3 (g) are mixed and allowed to reach equilibrium at 600 °C.

Determine the value of _K_c at 600 °C.
Question 2
HLPaper 1At 700 ºC, the equilibrium constant, _K_c, for the reaction is 1.075 × 108.
2H2 (g) + S2 (g) ⇌ 2H2S (g)
Which relationship is always correct for the equilibrium at this temperature?
Question 3
HLPaper 2Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) ⇌ (H2N)2CO(g) + H2O(g) Δ_H_ < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
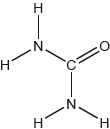
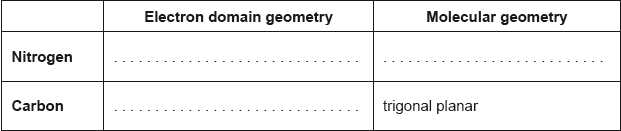
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
State the equilibrium constant expression, _K_c.
Predict, with a reason, the effect on the equilibrium constant, _K_c, when the temperature is increased.
Determine an approximate order of magnitude for _K_c, using sections 1 and 2 of the data booklet. Assume Δ_G_Θfor the forward reaction is approximately +50 kJ at 298 K.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
The mass spectrum of urea is shown below.
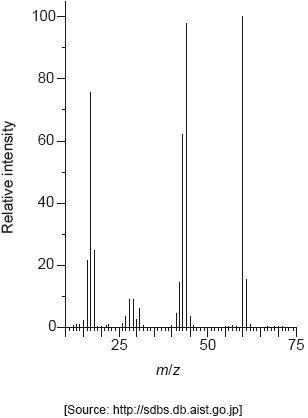
Identify the species responsible for the peaks at m/z = 60 and 44.

The IR spectrum of urea is shown below.
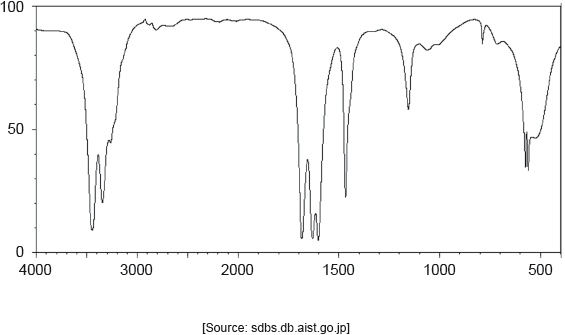
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.

Predict the number of signals in the 1H NMR spectrum of urea.
Predict the splitting pattern of the 1H NMR spectrum of urea.
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
Question 4
HLPaper 2White phosphorus is an allotrope of phosphorus and exists as P4.
An equilibrium exists between PCl3 and PCl5.
PCl3 (g) + Cl2 (g) ⇌ PCl5 (g)
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
a(i).
Write an equation for the reaction of white phosphorus (P4) with chlorine gas to form phosphorus trichloride (PCl3).
a(ii).
Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl bond angle in PCl3.

b(i).
Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.

b(ii).
Calculate the standard enthalpy change (Δ_H_⦵) for the forward reaction in kJ mol−1.
Δ_H_⦵f PCl3 (g) = −306.4 kJ mol−1
Δ_H_⦵f PCl5 (g) = −398.9 kJ mol−1
c(i).
Calculate the entropy change, Δ_S_, in J K−1 mol−1, for this reaction.

Chemistry 2e, Chpt. 21 Nuclear Chemistry, Appendix G: Standard Thermodynamic Properties for SelectedSubstanceshttps://openstax.org/books/chemistry-2e/pages/g-standard-thermodynamic-properties-for- selectedsubstances#page_667adccf-f900-4d86-a13d-409c014086ea © 1999-2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License. (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/.
c(ii).
Calculate the Gibbs free energy change (Δ_G_), in kJ mol−1, for this reaction at 25 °C. Use section 1 of the data booklet.
If you did not obtain an answer in c(i) or c(ii) use −87.6 kJ mol−1 and −150.5 J mol−1 K−1 respectively, but these are not the correct answers.
c(iii).
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use Δ_G_ = –43.5 kJ mol−1, but this is not the correct answer.
c(iv).
State the equilibrium constant expression, _K_c, for this reaction.
c(v).
State, with a reason, the effect of an increase in temperature on the position of this equilibrium.
c(vi).
Question 5
HLPaper 2This question is about iron.
Deduce the full electron configuration of Fe2+.
Explain why, when ligands bond to the iron ion causing the d-orbitals to split, the complex is coloured.
State the nuclear symbol notation, Z A X , for iron-54.
Mass spectrometry analysis of a sample of iron gave the following results:

Calculate the relative atomic mass, Ar, of this sample of iron to two decimal places.
An iron nail and a copper nail are inserted into a lemon.

Explain why a potential is detected when the nails are connected through a voltmeter.
Calculate the standard electrode potential, in V, when the Fe2+ (aq) | Fe (s) and Cu2+ (aq) | Cu (s) standard half-cells are connected at 298 K. Use section 24 of the data booklet.
f(i).
Calculate ΔGθ, in kJ, for the spontaneous reaction in (f)(i), using sections 1 and 2 of the data booklet.
f(ii).
Calculate a value for the equilibrium constant, Kc, at 298 K, giving your answer to two significant figures. Use your answer to (f)(ii) and section 1 of the data booklet.
(If you did not obtain an answer to (f)(ii), use −140 kJ mol−1, but this is not the correct value.)
f(iii).
Question 6
HLPaper 1Components X and Y are mixed together and allowed to reach equilibrium. The concentrations of X, Y, W and Z in the equilibrium mixture are 4, 1, 4 and 2 mol d m − 3 respectively.
X + 2Y ⇌ 2W + Z
What is the value of the equilibrium constant, _K_c?
Question 7
HLPaper 2Hydrogen and iodine react to form hydrogen iodide.
H2 (g) + I2 (g) ⇌ 2HI (g)
The following experimental data was obtained.

Consider the reaction of hydrogen with solid iodine.
H2 (g) + I2 (s) ⇌ 2HI (g) Δ_H_⦵= +53.0 kJ mol−1
Deduce the order of reaction with respect to hydrogen.
a(i).
Deduce the rate expression for the reaction.
a(ii).
Calculate the value of the rate constant stating its units.
a(iii).
State two conditions necessary for a successful collision between reactants.
State the equilibrium constant expression, _K_c, for this reaction.
Calculate the entropy change of reaction, Δ_S_⦵, in J K−1 mol−1.

d(i).
Predict, giving a reason, how the value of the ΔS⦵reaction would be affected if I2 (g) were used as a reactant.
d(ii).
Calculate the Gibbs free energy change, Δ_G_⦵, in kJ mol−1, for the reaction at 298 K. Use section 1 of the data booklet.
d(iii).
Calculate the equilibrium constant, _K_c, for this reaction at 298 K. Use your answer to (d)(iii) and sections 1 and 2 of the data booklet.
(If you did not obtain an answer to (d)(iii) use a value of 2.0 kJ mol−1, although this is not the correct answer).
d(iv).
Question 8
HLPaper 1Which is correct for a reaction with a positive change in Gibbs free energy, ΔGθ?
Question 9
HLPaper 2A mixture of 1.00 mol SO2(g), 2.00 mol O2(g) and 1.00 mol SO3(g) is placed in a 1.00 dm3container and allowed to reach equilibrium.
2SO2(g) + O2(g) ⇌2SO3(g)
Nitrogen oxide is in equilibrium with dinitrogen dioxide.
2NO(g) ⇌N2O2(g) Δ_H_Θ< 0
Deduce, giving a reason, the effect of increasing the temperature on theconcentration of N2O2.
A two-step mechanism is proposed for the formation of NO2(g) from NO(g) thatinvolves an exothermic equilibrium process.
First step: 2NO(g) ⇌N2O2(g) fast
Second step: N2O2(g) + O2 (g) → 2NO2(g) slow
Deduce the rate expression for the mechanism.
The rate constant for a reaction doubles when the temperature is increased from25.0 °C to 35 °C.
Calculate the activation energy, _E_a, in kJ mol−1 for the reaction using section 1 and 2 ofthe data booklet.
Question 10
HLPaper 2Many reactions are in a state of equilibrium.
The following reaction was allowed to reach equilibrium at 761 K.
H2(g) + I2(g) ⇌ 2HI (g) Δ_H_θ< 0
The pH of 0.010 mol dm–3 carbonic acid, H2CO3 (aq), is 4.17 at 25 °C.
H2CO3 (aq) + H2O (l) ⇌ HCO3– (aq) + H3O+ (aq).
State the equilibrium constant expression, _K_c , for this reaction.
The following equilibrium concentrations in mol dm–3 were obtained at 761 K.

Calculate the value of the equilibrium constant at 761 K.
Determine the value of Δ_G_θ, in kJ, for the above reaction at 761 K using section 1of the data booklet.
Calculate [H3O+] in the solution and the dissociation constant, _K_a , of the acid at25 °C.
Calculate _K_b for HCO3– acting as a base.
