
Topic 18 - Lewis acids and bases (HL)
Question 1
HLPaper 1Which combination of acid and base is most likely to have a pH of 8.5 at the equivalence point in a titration?
Question 2
HLPaper 2Magnetite, Fe3O4, is another ore of iron that contains both Fe2+ and Fe3+.
Iron exists as several isotopes.
Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
State the type of spectroscopy that could be used to determine their relative abundances.
b(i).
State the number of protons, neutrons and electrons in each species.

b(ii).
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
A voltaic cell is set up between the Fe2+ (aq) | Fe (s) and Fe3+ (aq) | Fe2+ (aq) half-cells.
Deduce the equation and the cell potential of the spontaneous reaction. Use section 24 of the data booklet.

The figure shows an apparatus that could be used to electroplate iron with zinc. Label the figure with the required substances.

Outline why, unlike typical transition metals, zinc compounds are not coloured.
Transition metals like iron can form complex ions. Discuss the bonding between transition metals and their ligands in terms of acid-base theory.
Question 3
HLPaper 2Butanoic acid, CH3CH2CH2COOH, is a weak acid and ethylamine, CH3CH2NH2, is a weak base.
State the equation for the reaction of each substance with water.

Draw a diagram showing the delocalization of electrons in the conjugate base of butanoic acid.
Deduce the average oxidation state of carbon in butanoic acid.
A 0.250 mol dm−3 aqueous solution of butanoic acid has a concentration of hydrogen ions, [H+], of 0.00192 mol dm−3. Calculate the concentration of hydroxide ions, [OH−], in the solution at 298 K.
Determine the pH of a 0.250 mol dm−3 aqueous solution of ethylamine at 298 K, using section 21 of the data booklet.
Sketch the pH curve for the titration of 25.0 cm3 of ethylamine aqueous solution with 50.0 cm3 of butanoic acid aqueous solution of equal concentration. No calculations are required.

Explain why butanoic acid is a liquid at room temperature while ethylamine is a gas at room temperature.
State a suitable reagent for the reduction of butanoic acid.
Deduce the product of the complete reduction reaction in (e)(i).
Question 4
HLPaper 2Copper forms two chlorides, copper(I) chloride and copper(II) chloride.
Two electrolysis cells were assembled using graphite electrodes and connected in series as shown.

Copper(I) chloride undergoes a disproportionation reaction, producing copper(II) chloride and copper.
2Cu+ (aq) → Cu (s) + Cu2+ (aq)
Dilute copper(II) chloride solution is light blue, while copper(I) chloride solution is colourless.
State the electron configuration of the Cu+ ion.
a(i).
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, Δ_H_θ, in kJ, for this reaction, using section 12 of the data booklet.
a(ii).
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label _E_a (cat).

a(iii).
Explain how the catalyst increases the rate of the reaction.
a(iv).
Solid copper(II) chloride absorbs moisture from the atmosphere to form a hydrate of formula CuCl2•xH2O.
A student heated a sample of hydrated copper(II) chloride, in order to determine the value of x. The following results were obtained:
Mass of crucible = 16.221 g
Initial mass of crucible and hydrated copper(II) chloride = 18.360 g
Final mass of crucible and anhydrous copper(II) chloride = 17.917 g
Determine the value of x.
State how current is conducted through the wires and through the electrolyte.
Wires:
Electrolyte:
c(i).
Write the half-equation for the formation of gas bubbles at electrode 1.
c(ii).
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
c(iii).
Deduce the half-equation for the formation of the gas identified in (c)(iii).
c(iv).
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24 of the data booklet.
e(i).
Comment on the spontaneity of the disproportionation reaction at 298 K.
e(ii).
Calculate the standard Gibbs free energy change, Δ_G_θ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
e(iii).
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
e(iv).
Deduce, giving a reason, the sign of the standard enthalpy change, Δ_H_θ, for the disproportionation reaction at 298 K.
e(v).
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
e(vi).
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
f(i).
Deduce why the Cu(I) solution is colourless.
f(ii).
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
f(iii).
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
f(iv).
Question 5
HLPaper 2When heated in air, magnesium ribbon reacts with oxygen to form magnesium oxide.
The reaction in (a)(i) was carried out in a crucible with a lid and the following data was recorded:
Mass of crucible and lid = 47.372 ±0.001 g
Mass of crucible, lid and magnesium ribbon before heating = 53.726 ±0.001 g
Mass of crucible, lid and product after heating = 56.941 ±0.001 g
When magnesium is burnt in air, some of it reacts with nitrogen to form magnesium nitride according to the equation:
3 Mg (s) + N2 (g) → Mg3N2 (s)
The presence of magnesium nitride can be demonstrated by adding water to the product. It is hydrolysed to form magnesium hydroxide and ammonia.
Most nitride ions are 14N3–.
Write a balanced equation for the reaction that occurs.
a(i).
Identify a metal, in the same period as magnesium, that does not form a basic oxide.
a(ii).
Calculate the amount of magnesium, in mol, that was used.
b(i).
Determine the percentage uncertainty of the mass of product after heating.
b(ii).
Assume the reaction in (a)(i) is the only one occurring and it goes to completion, but some product has been lost from the crucible. Deduce the percentage yield of magnesium oxide in the crucible.
b(iii).
Evaluate whether this, rather than the loss of product, could explain the yield found in (b)(iii).
c(i).
Suggest an explanation, other than product being lost from the crucible or reacting with nitrogen, that could explain the yield found in (b)(iii).
c(ii).
Calculate coefficients that balance the equation for the following reaction.

d(i).
Ammonia is added to water that contains a few drops of an indicator. Identify an indicator that would change colour. Use sections 21 and 22 of the data booklet.
d(ii).
Determine the oxidation state of nitrogen in Mg3N2 and in NH3.

d(iii).
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.

d(iv).
State the number of subatomic particles in this ion.

e(i).
Some nitride ions are 15N3–. State the term that describes the relationship between 14N3– and 15N3–.
e(ii).
The nitride ion and the magnesium ion are isoelectronic (they have the same electron configuration). Determine, giving a reason, which has the greater ionic radius.
e(iii).
Suggest, giving a reason, whether magnesium or nitrogen would have the greater sixth ionization energy.
e(iv).
Suggest two reasons why atoms are no longer regarded as the indivisible units of matter.
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.

Question 6
HLPaper 2Soluble acids and bases ionize in water.
A solution containing 0.510 g of an unknown monoprotic acid, HA, was titrated with0.100 mol dm–3 NaOH(aq). 25.0 cm3 was required to reach the equivalence point.
The following curve was obtained using a pH probe.

State, giving a reason, the strength of the acid.
State a technique other than a pH titration that can be used to detect theequivalence point.
Deduce the p_K_a for this acid.
The p_K_a of an anthocyanin is 4.35. Determine the pH of a 1.60 × 10–3 mol dm–3 solutionto two decimal places.
Question 7
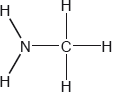
HLPaper 2Two hydrides of nitrogen are ammonia and hydrazine, N 2 H 4 . One derivative of ammonia is methanamine whose molecular structure is shown below.
Hydrazine is used to remove oxygen from water used to generate steam or hot water.
N 2 H 4 (aq) + O 2 (aq) → N 2 (g) + 2 H 2 O(l)
The concentration of dissolved oxygen in a sample of water is 8.0 × 10 − 3 g d m − 3 .
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
State the electron domain geometry around the nitrogen atom and its hybridization inmethanamine.
Ammonia reacts reversibly with water.
N H 3 (g) + H 2 O(l) ⇌ NH 4 + (aq) + O H − (aq)
Explain the effect of adding H + (aq) ions on the position of the equilibrium.
Hydrazine reacts with water in a similar way to ammonia. (The association of amolecule of hydrazine with a second H+ is so small it can be neglected.)
N 2 H 4 (aq) + H 2 O(l) ⇌ N 2 H 5 + (aq) + O H − (aq)
p K b (hydrazine) = 5.77
Calculate the pH of a 0.0100 mol d m − 3 solution of hydrazine.
Suggest a suitable indicator for the titration of hydrazine solution with dilutesulfuric acid using section 22 of the data booklet.
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
Determine the enthalpy change of reaction, Δ H, in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
N 2 H 4 (g) → N 2 (g) + 2 H 2 (g)
The standard enthalpy of formation of N 2 H 4 (l) is + 50.6 kJ mo l − 1 . Calculate the enthalpy of vaporization, Δ H vap , of hydrazine in kJ mo l − 1 . N 2 H 4 (l) → N 2 H 4 (g) (If you did not get an answer to (f), use − 85 kJ but this is not the correct answer.)
Calculate, showing your working, the mass of hydrazine needed to remove all the dissolved oxygen from 1000 d m 3 of the sample.
Calculate the volume, in d m 3 , of nitrogen formed under SATP conditions. (The volume of 1 mol of gas = 24.8 d m 3 at SATP.)
Question 8
HLPaper 1Which of the following will form a buffer solution if combined in appropriate molar ratios?
Question 9
SLPaper 1Which is amphiprotic?
Question 10
HLPaper 1Where is the buffer region for the titration of a weak acid with a strong base?

