
Topic 13 - Periodicity (HL)
Question 1
HLPaper 2Magnetite, Fe3O4, is another ore of iron that contains both Fe2+ and Fe3+.
Iron exists as several isotopes.
Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
State the type of spectroscopy that could be used to determine their relative abundances.
b(i).
State the number of protons, neutrons and electrons in each species.

b(ii).
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
A voltaic cell is set up between the Fe2+ (aq) | Fe (s) and Fe3+ (aq) | Fe2+ (aq) half-cells.
Deduce the equation and the cell potential of the spontaneous reaction. Use section 24 of the data booklet.

The figure shows an apparatus that could be used to electroplate iron with zinc. Label the figure with the required substances.

Outline why, unlike typical transition metals, zinc compounds are not coloured.
Transition metals like iron can form complex ions. Discuss the bonding between transition metals and their ligands in terms of acid-base theory.
Question 2
HLPaper 1What is the correct explanation for the colour of [Cu(H2O)6]2+?
Question 3
HLPaper 2Copper forms two chlorides, copper(I) chloride and copper(II) chloride.
Two electrolysis cells were assembled using graphite electrodes and connected in series as shown.

Copper(I) chloride undergoes a disproportionation reaction, producing copper(II) chloride and copper.
2Cu+ (aq) → Cu (s) + Cu2+ (aq)
Dilute copper(II) chloride solution is light blue, while copper(I) chloride solution is colourless.
State the electron configuration of the Cu+ ion.
a(i).
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, Δ_H_θ, in kJ, for this reaction, using section 12 of the data booklet.
a(ii).
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label _E_a (cat).

a(iii).
Explain how the catalyst increases the rate of the reaction.
a(iv).
Solid copper(II) chloride absorbs moisture from the atmosphere to form a hydrate of formula CuCl2•xH2O.
A student heated a sample of hydrated copper(II) chloride, in order to determine the value of x. The following results were obtained:
Mass of crucible = 16.221 g
Initial mass of crucible and hydrated copper(II) chloride = 18.360 g
Final mass of crucible and anhydrous copper(II) chloride = 17.917 g
Determine the value of x.
State how current is conducted through the wires and through the electrolyte.
Wires:
Electrolyte:
c(i).
Write the half-equation for the formation of gas bubbles at electrode 1.
c(ii).
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
c(iii).
Deduce the half-equation for the formation of the gas identified in (c)(iii).
c(iv).
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24 of the data booklet.
e(i).
Comment on the spontaneity of the disproportionation reaction at 298 K.
e(ii).
Calculate the standard Gibbs free energy change, Δ_G_θ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
e(iii).
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
e(iv).
Deduce, giving a reason, the sign of the standard enthalpy change, Δ_H_θ, for the disproportionation reaction at 298 K.
e(v).
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
e(vi).
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
f(i).
Deduce why the Cu(I) solution is colourless.
f(ii).
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
f(iii).
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
f(iv).
Question 4
HLPaper 1Which is correct for the complex ion in [Fe(H2O)5Cl]SO4?

Question 5
HLPaper 3Alloys containing at least 60 % copper reduce the presence of bacteria on their surface.The percentage of copper in brass, an alloy of copper and zinc, can be determined by UV-vis spectrometry.
A sample of brass is dissolved in concentrated nitric acid and then made up to 250.0 cm3with water before analysis.
Cu (s) + 4HNO3(aq) → Cu(NO3)2(aq) + 2NO2(g) + 2H2O (l)
3Zn (s) + 8HNO3(aq) → 3Zn(NO3)2(aq) + 2NO (g) + 4H2O (l)
The concentration of copper(II) ions in the resulting solution is then determined from a calibration curve, which is plotted by measuring the light absorbance of standard solutions.

You may find the following chart and diagram helpful.


Outline why the initial reaction should be carried out under a fume hood.
Deduce the equation for the relationship between absorbance and concentration.

Copper(II) ion solutions are blue. Suggest, giving your reason, a suitable wavelength of light for the analysis.
Outline how a solution of 0.0100 mol dm−3 is obtained from a standard 1.000 mol dm−3 copper(II) sulfate solution, including two essential pieces of glassware you would need.
The original piece of brass weighed 0.200 g. The absorbance was 0.32.
Calculate, showing your working, the percentage of copper by mass in the brass.
Deduce the appropriate number of significant figures for your answer in (e)(i).
Comment on the suitability of using brass of this composition for door handles in hospitals.
If you did not obtain an answer to (e)(i), use 70 % but this is not the correct answer.
Suggest another property of brass that makes it suitable for door handles.
Titration is another method for analysing the solution obtained from adding brass to nitric acid.
Copper(II) ions are reduced to copper(I) iodide by the addition of potassium iodide solution, releasing iodine that can be titrated with sodium thiosulfate solution, Na2S2O3(aq). Copper(I) iodide is a white solid.
4I−(aq) + 2Cu2+(aq) → 2CuI (s) + I2(aq)
I2(aq) + 2S2O32−(aq) → 2I−(aq) + S4O62−(aq)
Suggest why the end point of the titration is difficult to determine, even with the addition of starch to turn the remaining free iodine black.
Question 6
HLPaper 2The properties of elements can be predicted from their position in the periodic table.
Explain why Si has a smaller atomic radius than Al.
a(i).
Explain why the first ionization energy of sulfur is lower than that of phosphorus.
a(ii).
State the condensed electron configurations for Cr and Cr3+.

b(i).
Describe metallic bonding and how it contributes to electrical conductivity.
b(ii).
Deduce, giving a reason, which complex ion [Cr(CN)6]3− or [Cr(OH)6]3− absorbs higher energy light. Use section 15 of the data booklet.
b(iii).
[Cr(OH)6]3− forms a green solution. Estimate a wavelength of light absorbed by this complex, using section 17 of the data booklet.
b(iv).
Deduce the Lewis (electron dot) structure and molecular geometry of sulfurtetrafluoride, SF4, and sulfur dichloride, SCl2.

Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
Question 7
HLPaper 2Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) ⇌ (H2N)2CO(g) + H2O(g) Δ_H_ < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
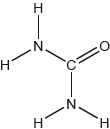
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
State the equilibrium constant expression, _K_c.
Predict, with a reason, the effect on the equilibrium constant, _K_c, when the temperature is increased.
Determine an approximate order of magnitude for _K_c, using sections 1 and 2 of the data booklet. Assume Δ_G_Θfor the forward reaction is approximately +50 kJ at 298 K.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
The mass spectrum of urea is shown below.
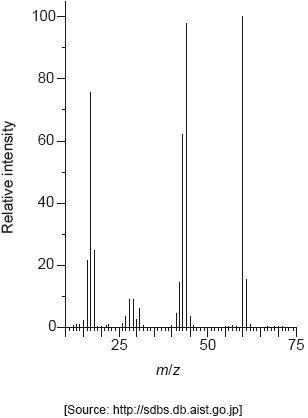
Identify the species responsible for the peaks at m/z = 60 and 44.

The IR spectrum of urea is shown below.
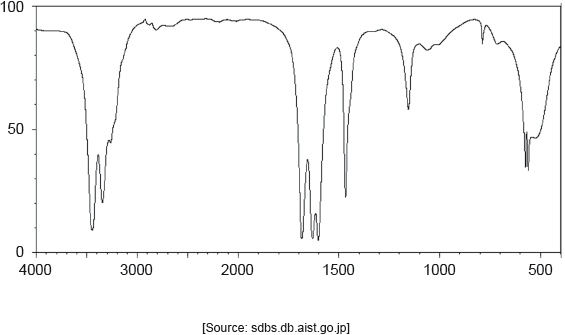
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.

Predict the number of signals in the 1H NMR spectrum of urea.
Predict the splitting pattern of the 1H NMR spectrum of urea.
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
Question 8
HLPaper 1What is the charge on the iron(III) complex ion in [Fe(OH)2(H2O)4]Br?
Question 9
HLPaper 1How is colour produced in transition metal complexes?
Question 10
HLPaper 2Cobalt forms the transition metal complex [Co(NH3)4 (H2O)Cl]Br.
Trends in physical and chemical properties are useful to chemists.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down thegroup whereas the melting points of the group 17 elements (F → I) increase down thegroup.

State the shape of the complex ion.
Deduce the charge on the complex ion and the oxidation state of cobalt.

Describe, in terms of acid-base theories, the type of reaction that takes place betweenthe cobalt ion and water to form the complex ion.
