
Topic 12 - The periodic table the transition metals (HL)
Question 1
HLPaper 1Which statement explains one of the decreases in first ionization energy (I.E.) across period 3?

Question 2
HLPaper 1Which transition on the diagram corresponds to the ionization of hydrogen in the ground state?
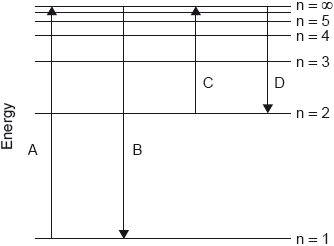
Question 3
HLPaper 2When heated in air, magnesium ribbon reacts with oxygen to form magnesium oxide.
The reaction in (a)(i) was carried out in a crucible with a lid and the following data was recorded:
Mass of crucible and lid = 47.372 ±0.001 g
Mass of crucible, lid and magnesium ribbon before heating = 53.726 ±0.001 g
Mass of crucible, lid and product after heating = 56.941 ±0.001 g
When magnesium is burnt in air, some of it reacts with nitrogen to form magnesium nitride according to the equation:
3 Mg (s) + N2 (g) → Mg3N2 (s)
The presence of magnesium nitride can be demonstrated by adding water to the product. It is hydrolysed to form magnesium hydroxide and ammonia.
Most nitride ions are 14N3–.
Write a balanced equation for the reaction that occurs.
a(i).
Identify a metal, in the same period as magnesium, that does not form a basic oxide.
a(ii).
Calculate the amount of magnesium, in mol, that was used.
b(i).
Determine the percentage uncertainty of the mass of product after heating.
b(ii).
Assume the reaction in (a)(i) is the only one occurring and it goes to completion, but some product has been lost from the crucible. Deduce the percentage yield of magnesium oxide in the crucible.
b(iii).
Evaluate whether this, rather than the loss of product, could explain the yield found in (b)(iii).
c(i).
Suggest an explanation, other than product being lost from the crucible or reacting with nitrogen, that could explain the yield found in (b)(iii).
c(ii).
Calculate coefficients that balance the equation for the following reaction.

d(i).
Ammonia is added to water that contains a few drops of an indicator. Identify an indicator that would change colour. Use sections 21 and 22 of the data booklet.
d(ii).
Determine the oxidation state of nitrogen in Mg3N2 and in NH3.

d(iii).
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.

d(iv).
State the number of subatomic particles in this ion.

e(i).
Some nitride ions are 15N3–. State the term that describes the relationship between 14N3– and 15N3–.
e(ii).
The nitride ion and the magnesium ion are isoelectronic (they have the same electron configuration). Determine, giving a reason, which has the greater ionic radius.
e(iii).
Suggest, giving a reason, whether magnesium or nitrogen would have the greater sixth ionization energy.
e(iv).
Suggest two reasons why atoms are no longer regarded as the indivisible units of matter.
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.

Question 4
HLPaper 2The properties of elements can be predicted from their position in the periodic table.
Explain why Si has a smaller atomic radius than Al.
a(i).
Explain why the first ionization energy of sulfur is lower than that of phosphorus.
a(ii).
State the condensed electron configurations for Cr and Cr3+.

b(i).
Describe metallic bonding and how it contributes to electrical conductivity.
b(ii).
Deduce, giving a reason, which complex ion [Cr(CN)6]3− or [Cr(OH)6]3− absorbs higher energy light. Use section 15 of the data booklet.
b(iii).
[Cr(OH)6]3− forms a green solution. Estimate a wavelength of light absorbed by this complex, using section 17 of the data booklet.
b(iv).
Deduce the Lewis (electron dot) structure and molecular geometry of sulfurtetrafluoride, SF4, and sulfur dichloride, SCl2.

Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
Question 5
HLPaper 1The diagram shows the first ionisation energies of consecutive elements in the same period of the periodic table.

Which factor explains why element X has a higher first ionisation energy than element Y?
Question 6
HLPaper 2Dinitrogen monoxide, N2O, causes depletion of ozone in the stratosphere.
Different sources of N2O have different ratios of 14N : 15N.
The Lewis (electron dot) structure of the dinitrogen monoxide molecule can be represented as:

Outline why ozone in the stratosphere is important.
a(i).
Dinitrogen monoxide in the stratosphere is converted to nitrogen monoxide, NO (g).
Write two equations to show how NO (g) catalyses the decomposition of ozone.
a(ii).
State one analytical technique that could be used to determine the ratio of 14N : 15N.
b(i).
A sample of gas was enriched to contain 2 % by mass of 15N with the remainder being 14N.
Calculate the relative molecular mass of the resulting N2O.
b(ii).
Predict, giving two reasons, how the first ionization energy of 15N compares with that of 14N.
b(iii).
Explain why the first ionization energy of nitrogen is greater than both carbon and oxygen.
Nitrogen and carbon:
Nitrogen and oxygen:
State what the presence of alternative Lewis structures shows about the nature of the bonding in the molecule.
d(i).
State, giving a reason, the shape of the dinitrogen monoxide molecule.
d(ii).
Deduce the hybridization of the central nitrogen atom in the molecule.
d(iii).
Question 7
HLPaper 2Magnesium is a group 2 metal which exists as a number of isotopes and forms many compounds.
Magnesium ions produce no emission or absorption lines in the visible region of the electromagnetic spectrum. Suggest why most magnesium compounds tested in a school laboratory show traces of yellow in the flame.
(i) Explain the convergence of lines in a hydrogen emission spectrum.
(ii) State what can be determined from the frequency of the convergence limit.
Magnesium chloride can be electrolysed.
(i) Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922K and 987K respectively.

(ii) Identify the type of reaction occurring at the cathode (negative electrode).
(iii) State the products when a very dilute aqueous solution of magnesium chloride is electrolysed.

Standard electrode potentials are measured relative to the standard hydrogen electrode. Describe a standard hydrogen electrode.
A magnesium half-cell, Mg(s)/Mg2+(aq), can be connected to a copper half-cell, Cu(s)/Cu2+(aq).
(i) Formulate an equation for the spontaneous reaction that occurs when the circuit is completed.
(ii) Determine the standard cell potential, in V, for the cell. Refer to section 24 of the data booklet.
(iii) Predict, giving a reason, the change in cell potential when the concentration of copper ions increases.
Question 8
HLPaper 2Properties of elements and their compounds can be related to the position of the elements in the periodic table.
Explain the decrease in atomic radius from Na to Cl.
Explain why the radius of the sodium ion, Na+, is smaller than the radius of the oxide ion, O2−.
Sketch a graph to show the relative values of the successive ionization energiesof boron.

Predict, giving your reasons, whether Mn2+ or Fe2+ is likely to have a more exothermic enthalpy of hydration.
Question 9
HLPaper 1The graph shows the first ionization energies of some consecutive elements.
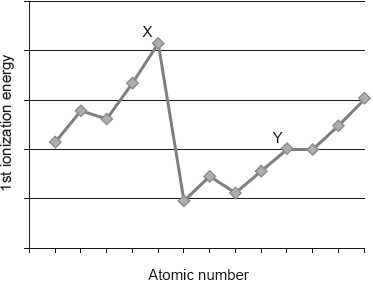
Which statement is correct?
Question 10
HLPaper 1Which of the following transitions in the hydrogen atom emits the least energy?
