
Topic 12 - Quantum & Nuclear Physics
Question 1
HLPaper 1In a photoelectric experiment a stopping voltage required to prevent photoelectrons from flowing across the photoelectric cell is measured for light of two frequencies and . The results obtained are shown. The ratio is an estimate of
The ratio is an estimate of
Question 2
HLPaper 1A particle is confined within a nucleus. What is the order of magnitude of the uncertainty in the momentum of the particle?
Question 3
HLPaper 2Hydrogen atoms in an ultraviolet (UV) lamp make transitions from the first excited state to the ground state. Photons are emitted and are incident on a photoelectric surface as shown.
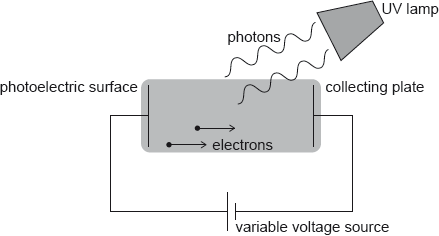
The photons cause the emission of electrons from the photoelectric surface. The work function of the photoelectric surface is 5.1 eV.
The electric potential of the photoelectric surface is 0 V. The variable voltage is adjusted so that the collecting plate is at V.
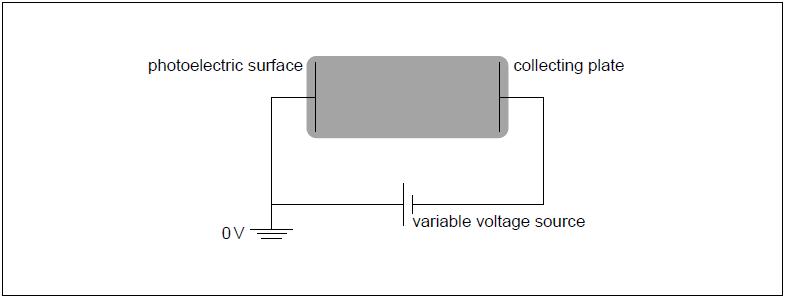
Show that the energy of photons from the UV lamp is about .
Calculate, in J, the maximum kinetic energy of the emitted electrons.
Suggest, with reference to conservation of energy, how the variable voltage source can be used to stop all emitted electrons from reaching the collecting plate.
The variable voltage can be adjusted so that no electrons reach the collecting plate. Write down the minimum value of the voltage for which no electrons reach the collecting plate.
On the diagram, draw and label the equipotential lines at V and V.
An electron is emitted from the photoelectric surface with kinetic energy eV. Calculate the speed of the electron at the collecting plate.
Question 4
HLPaper 1An electron of mass m has an uncertainty in its position r. What is the uncertainty in the speed of this electron?
Question 5
HLPaper 2In a classical model of the singly-ionized helium atom, a single electron orbits the nucleus in a circular orbit of radius r.

The Bohr model for hydrogen can be applied to the singly-ionized helium atom. In this model the radius* , in m, of the orbit of the electron is given by -11 ×
Show that the speed of the electron with mass , is given by a(i).
Hence, deduce that the total energy of the electron is given by .
a(ii).
In this model the electron loses energy by emitting electromagnetic waves. Describe the predicted effect of this emission on the orbital radius of the electron. a(iii).
Show that the de Broglie wavelength of the electron in the state is m.
The formula for the de Broglie wavelength of a particle is . b(i).
Estimate for , the ratio .
State your answer to one significant figure.
b(ii).
The description of the electron is different in the Schrodinger theory than in the Bohr model. Compare and contrast the description of the electron according to the Bohr model and to the Schrodinger theory.
Question 6
HLPaper 1Some of the nuclear energy levels of oxygen-14 (O) and nitrogen-14 (N) are shown.
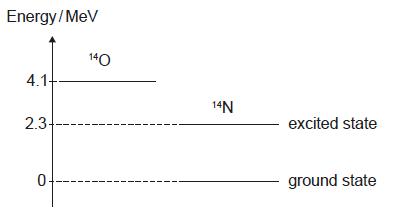
A nucleus of decays into a nucleus of with the emission of a positron and a gamma ray. What is the maximum energy of the positron and the energy of the gamma ray?
| Maximum energy of the positron/MeV | Energy of the gamma ray /MeV | |
|---|---|---|
| A. | 1.8 | 2.3 |
| B. | 1.8 | 4.1 |
| C. | 2.3 | 1.8 |
| D. | 4.1 | 2.3 |
Question 7
HLPaper 1When monochromatic light is incident on a metallic surface, electrons are emitted from the surface. The following changes are considered.
- I. Increase the intensity of the incident light
- II. Increase the frequency of light
- III. Decrease the work function of the surface
Which changes will result in electrons of greater energy being emitted from the surface?
Question 8
HLPaper 1A radioactive element has decay constant (expressed in s^-1). The number of nuclei of this element at t = 0 is N.
What is the expected number of nuclei that will have decayed after 1 s?
Question 9
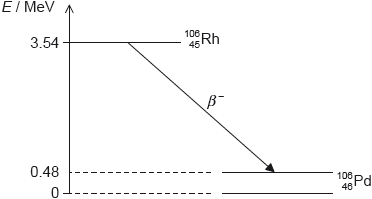
HLPaper 2Rhodium-106 () decays into palladium-106 () by beta minus () decay. The diagram shows some of the nuclear energy levels of rhodium-106 and palladium-106. The arrow represents the decay.
Bohr modified the Rutherford model by introducing the condition mvr = n. Outline the reason for this modification.
Show that the speed v of an electron in the hydrogen atom is related to the radius r of the orbit by the expression where k is the Coulomb constant.
Using the answer in (b) and (c)(i), deduce that the radius r of the electron’s orbit in the ground state of hydrogen is given by the following expression.
Calculate the electron's orbital radius in .
Explain what may be deduced about the energy of the electron in the decay.
Suggest why the decay is followed by the emission of a gamma ray photon.
Question 10
HLPaper 1In the Bohr model for hydrogen an electron in the ground state has orbit radius r and speed v. In the first excited state the electron has orbit radius 4r. What is the speed of the electron in the first excited state?
